magnesium sulfate can be made by reacting magnesium metal with which acid?
Magnesium metal reacts fairly slowly with concentrated sulfuric acid. _____ acid 1 ii Use the equation to help you to describe what you would observe when magnesium reacts with the acid.
Synthetically prepared magnesium sulfate is sold as Epsom salt MgSO 4.
. As the reacting acid is hydrochloric acid then the salt produced will be a chloride. 8 Magnesium carbonate reacts with dilute hydrochloric acid. Magnesium sulfate MgSO 4 is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. Mgs H 2 SO 4 aq MgSO 4 aq H 2 g.
They may be recrystallised to increase purity. When you want magnesium sulfate salts in lab use magnesium oxide and magnesium carbonate to prepare magnesium sulfate salts because magnesium reacts with sulfuric acids to produce hydrogen gas and that is very dangerous because it explode easily. Taking a closer look at two of the metals commonly reacted with acids. Acids take part in reactions in which salts are produced.
1 A flammable gas is produced in reaction P. P magnesium dilute hydrochloric acid Q zinc oxide dilute sulfuric acid R sodium hydroxide dilute hydrochloric acid S copper carbonate dilute sulfuric acid Which statements about the products of the reactions are correct. Magnesium reacts with hydrochloric acid according to the equation. A Describe how you could make a solution of magnesium sulphate starting with magnesium oxide powder and dilute sulphuric acid.
The total volume of gas collected was recorded every 20 seconds. The reaction produces hydrogen gas. The volume of the hydrogen gas produced will be measured at room temperature and pressure. Acid Alkali Salt Water Making Soluble Salts from acids and bases Salts can be made by reacting an acid with a insoluble base.
A hydrate form of magnesium sulfate called kieserite MgSO 4 H 2 O occurs as a mineral deposit. Both processes produced magnesium amalgam from which he made the metal. MgOs H 2SO 4aq MgSO 4aq Magnesium sulfate7-water MgSO-47H. Titration must be used if the reactants are soluble.
In 1808 Humphry Davy took moistened magnesium sulfate and electrolyzed it onto a mercury cathode. The solid is added to the acid until no more reacts and the excess solid is filtered off to produce a solution. MgCO3s 2HCl aq MgCl 2aq H2Ol CO2g An excess of magnesium carbonate pieces was added to dilute hydrochloric acid. Acid Bases Salt Water Making Soluble Salts from acids and metal carbonates Salts.
Mgs H2SO4aq MgSO4aq H2g i Name the acid used to make magnesium sulfate. It can be prepared by the reaction of magnesium metal with an acid. You can make magnesium sulfate7-water in the laboratory by reacting magnesium - oxide with dilute sulfuric acid. Mg s 2 HCl aq -- MgCl2aq H2g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gas.
Magnesium sulfate can be made by reacting magnesium metal with an acid. As the salt produced is a sulphate the reacting acid must be sulphuric acid In this type of reaction an acid reacts with a carbonate to give a salt water and carbon dioxide. The apparatus in the diagram was used to measure the volume of gas produced. Magnesium reacts with dilute sulfuric acid to give a colourless gas hydrogen and a colourless solution of magnesium sulfate.
The magnesium chloride at these sources still contains significant amounts of water and must be dried in order to make the magnesium chloride anhydrous before it can be electrolyzed to produce metal. 2 Water is formed in all reactions. 3 All the salts formed are soluble in water. In this type of reaction an acid reacts with a metal to produce a salt and hydrogen.
Insoluble salts are made by precipitation reactions. Metal metal oxide and metal carbonate reacts with acids to produce salts. 7 A solution of magnesium sulphate can be made by reacting magnesium oxide with warm sulphuric acid. Soluble salts can be made by reacting acids with soluble or insoluble reactants.
O crystals are obtained by evaporation. At the end of the video a comparison is made betwe. Magnesium sulfate is usually obtained directly from dry lake beds and other natural sources. Anhydrous magnesium sulfate was reported from some burning coal dumps.
The equation for the reaction of magnesium with this acid is. A gas is also produced. A gas is also produced. Many combinations of compounds could make magnesium sulfatemagnesium hydroxide and sulfuric acidmagnesium oxide and sulfuric acidmagnesium hydroxide and sulfur trioxideetc.
The Reaction of Magnesium with Hydrochloric Acid In this experiment you will determine the volume of the hydrogen gas that is produced when a sample of magnesium reacts with hydrochloric acid. Magnesium sulfate isMgSO4Making Soluble Salts from acids and alkalis Salts can be made by reacting an acid with an alkali. Magnesium is also extracted from salt brines which contains about 10 percent magnesium chloride. 3 b Describe how you would obtain pure dry crystals of hydrated magnesium sulphate MgSO 47H.
Soluble salts can be made from acids by reacting them with solid insoluble substances such as metals metal oxides hydroxides or carbonates. He also converted red hot magnesium oxide with potassium vapor collecting the magnesium into mercury. It can also be prepared by reacting magnesite magnesium carbonate MgCO 3 or magnesia oxide MgO with sulfuric acid. In either case the oxide is reacted with sulfuric acid toproduce magnesium sulphate.
Hydrogen chemists usually make salts by reacting a metal compound such as a metal carbonate with an acid. Correct answer to the question Magnesium sulfate can be made by reacting magnesium metal with an acid. The data you obtain will enable you to answer the question.

The Rate Of Reaction Of Magnesium With Hydrochloric Acid Experiment Rsc Education
How Does Magnesium React With Nitric Acid Quora
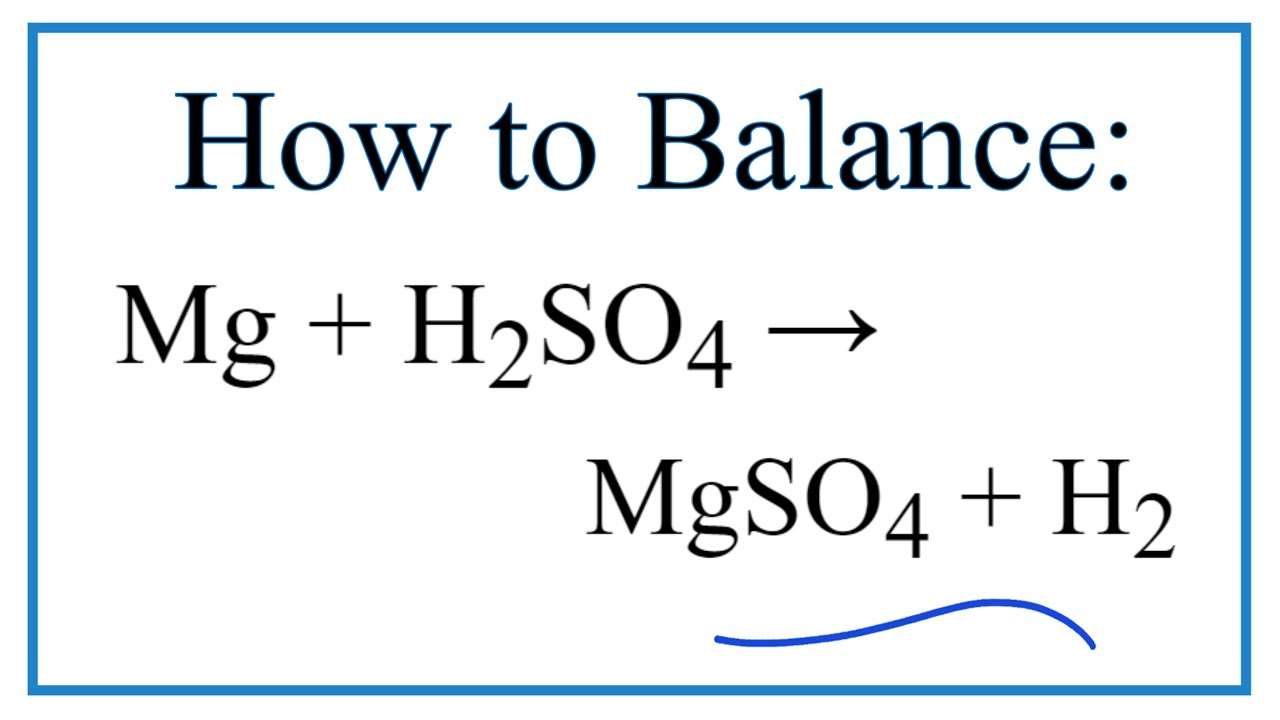
How To Balance Mg H2so4 Mgso4 H2 Magnesium Dilute Sulfuric Acid Youtube

Chemistry Education에 있는 Mrs Calverley님의 핀
When Is Magnesium Added To Sulphuric Acid Energy Absorbed Or Evolved Quora

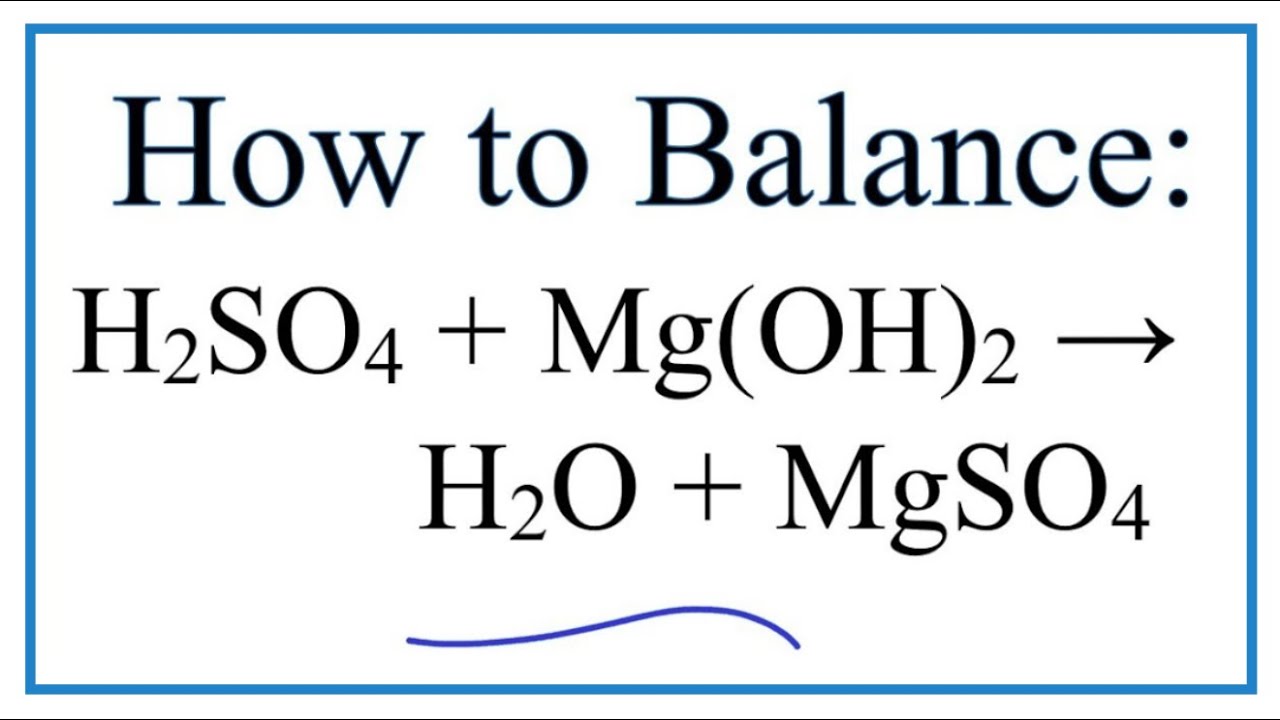
How To Balance H2so4 Mg Oh 2 H2o Mgso4 Sulfuric Acid Magnesium Hydroxide Youtube
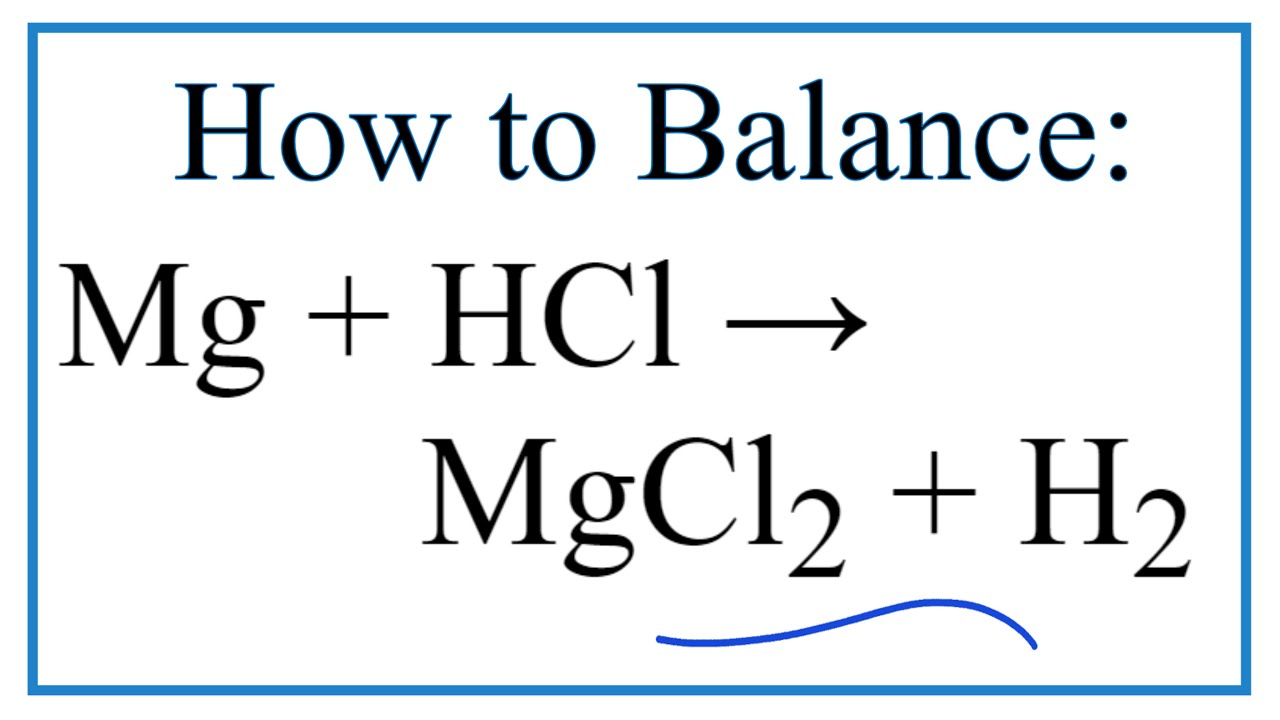
How To Balance Mg Hcl Mgcl2 H2 Magnesium Hydrochloric Acid Youtube
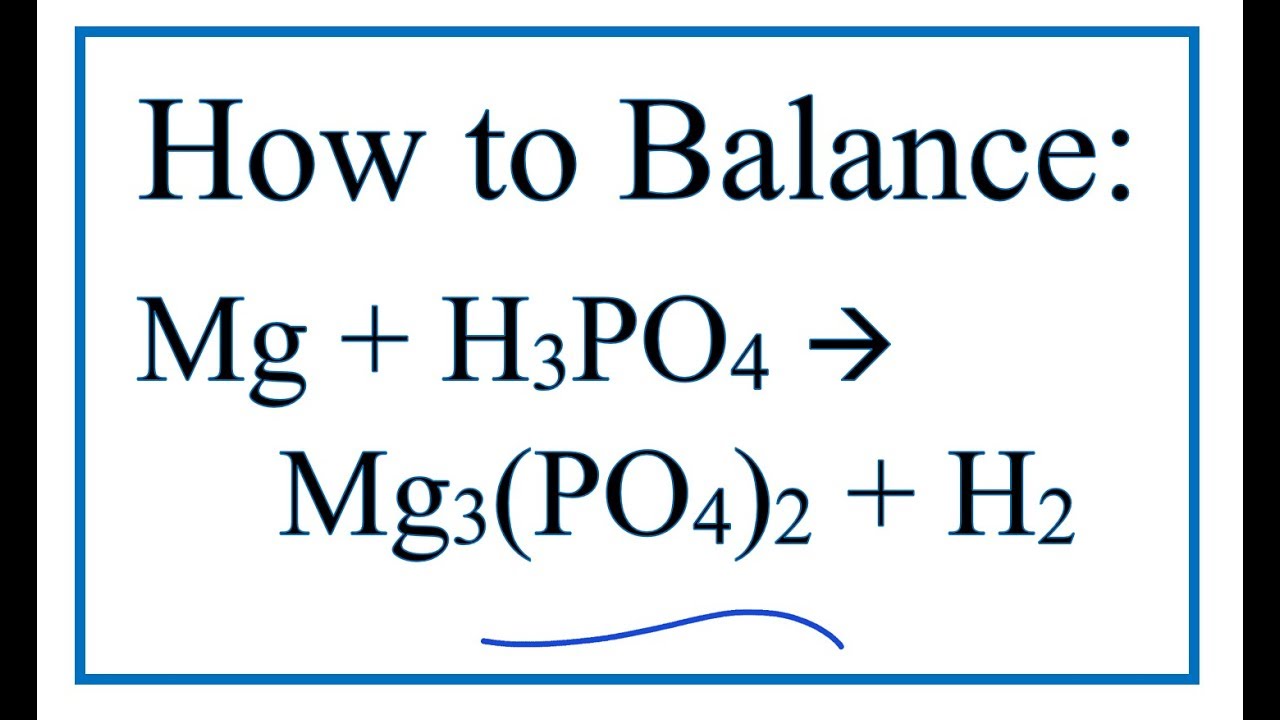
How To Balance Mg H3po4 Mg3 Po4 2 H2 Magnesium Phosphoric Acid Youtube

Posting Komentar untuk "magnesium sulfate can be made by reacting magnesium metal with which acid?"